Abstract
Research towards the application of hydrogen peroxide in bi-propellant thruster systems is of great interest to the aerospace community for its non-toxicity and low cost. Because Hydrogen Peroxide’s decomposition into Hydrogen, Water, and Oxygen is an exothermic reaction. Researchers theorize that hydrogen peroxide decomposing within a combustion generates higher temperatures and it is therefore a more effective method of oxidation for rocket engines. This study shows that decomposition of hydrogen peroxide must happen prior to combustion to maximize temperature within a propulsion system.
Nomenclature
F = force in newtons
Ṁ = mass flow rate in grams / seconds
V = velocity
P = pressure
A = area
T = temperature in Celsius
Subscripts
e = exit of nozzle
t = throat of nozzle
Introduction
Hydrogen peroxide, H2O2, is a powerful albeit an unstable oxidizer. Under normal conditions, H2O2 will decompose under heat and UV light [1] with the following equation:
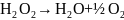
Because of its low toxicity rating and low cost of production, peroxide has been used as propellant for rockets since the mid-1950s [2]. When used as a monopropellant hydrogen peroxide passes through a catalyst causing it to decompose rapidly, releasing thermal energy. The catalyst for hydrogen peroxide is usually a superposed silver mesh [3]. It has the ability to rapidly decompose peroxide while not disintegrating.
There is an increased interest in using hydrogen peroxide as an oxidizer for hybrid rocket engine designs because of how it is more environmentally friendly compared to other oxidizers like hydrazine [4]. These hybrid rocket engine designs are bi-propellent systems. They use one propellant that is a solid and another that is liquid, a catalyst rapidly decomposes the peroxide prior to introduction in the combustion chamber.
By applying the fundamentals of the ideal gas law, the thrust of a rocket is produced from the mass being expelled from combustion chamber of the rocket. The products of the combustion are a gas and considered a working fluid that accelerate towards the exhaust. Because of Newton’s third law of motion, the rapid expulsion of mass from the rear of the rocket causes the rocket to accelerate [5].
Since the general thrust equation is:
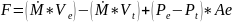
Where F is force in Newtons, Ṁ represents the mass flow rate in g/s, V is velocity in m/s, Pe is pressure in bars at the exit of the nozzle, Pt is pressure in bars at the throat of the nozzle, and Ae is the area in cm at the exit of the nozzle bell. Through the general thrust equation, it is possible to calculate how to control the pressure within the combustion chamber by increasing temperature.
Given the Equation of State:
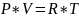
Where P is Pressure, V is Volume, R is the Gas Constant, and T is Temperature. The equation can be re-balanced to the following:
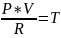
This rebalanced equation displays the relation of temperature to pressure. Since the gas constant and volume of the combustion chamber are constants, temperature is the only other variable in the gas constant law available to manipulate pressure, and ultimately thrust of the rocket.
In this study, the most efficient oxidation is determined by the method of introduction which produces the highest maximum temperatures within the combustion.
Though many hybrid rocket designs state the advantage of using a catalyst to rapidly decompose peroxide and in introducing H2O + O2 into the combustion chamber, this study explores when peroxide should be introduced into the combustion process to maximize enthalpy.
II – Method
In this study, it is proposed that atomizing hydrogen peroxide is the most efficient way to introduce it to the combustion process. Hydrogen peroxide when atomized creates an exothermic reaction resulting in higher temperatures. The research therefore compares the application of a 3% hydrogen peroxide solution as an oxidizer through atomization and resulting decomposition. The combustion fuel used in this study is 2 g (± 0.1) of Hexamethylenetetramine (CH2)6N4, chosen because of its high energy content, stability, and relative ease of production [6]. All (CH2)6N4 comes from the same lot from a sole source manufacturer.
The following calculation demonstrates the combustion of Hexamethylenetetramine under ideal circumstances and do not account for the surrounding atmosphere nor the dilution of the hydrogen peroxide used:
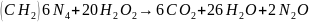
Since this study utilizes a low concentration, changes in temperature will be minor. Slight changes in temperature during the experiment will translate to larger changes when higher concentrations of H2O2 are used.
Data is gathered by measuring the combustion temperature of three samples in a control sample burn, three samples with decomposed hydrogen peroxide, and three samples with atomized hydrogen peroxide.
In all variants of the experiment Hexamethylenetetramine is placed on an aluminum test stand with holes cut through it in a ‘star pattern’. The temperature in this experiment is measured using a Voltcraft IR-Thermometer 260-8S with a duration of twenty seconds for each sample. Because the measurements are taken with an IR thermometer each reading is centered on the burning Hexamethylenetetramine cube and the thermometer is held approximately 25 cm away from the flame.
Each sample undergoes the following cycle:
- Measure start temp of cube prior to combustion.
- Ignite fuel.
- Allow fuel to burn for 10 sec to allow fuel to reach max burn temperature.
- Introduce oxidizer (if applicable).
- Measure temperature of fuel for 20 sec.
- Record maximum and minimum temperatures.
- Extinguish flame.
Samples are discarded after combustion allowing each sample to combust with the same mass.
A. Introduction of Atomized Hydrogen Peroxide to Combustion
To introduce atomized hydrogen peroxide, the study uses a pneumatic atomizing nozzle with a reciprocating pump mechanism. The nozzle is kept approximately 17 cm away from the flame and sprayed every three seconds allowing the mist to interact with the combustion.
B. Introduction of Decomposed Hydrogen Peroxide to Combustion
Decomposed hydrogen peroxide is introduced to the combustion by mixing 15 ml of H2O2 with 5 ml of a yeast enzyme in a bowl. The bowl is then placed underneath the combustion; as the O2 from the decomposed hydrogen rises it interacts with the combustion of (CH2)6N4. This study chose to use a yeast enzyme over magnesium dioxide or other inorganic material to better understand the possibility of using organic oxidizer decomposition, or more environmentally-friendly, rocket propulsion designs.
The ambient temperature of H2O2 in a bowl during the experiment is 25.0 °C. After the catalytic enzyme is added to the bowl of H2O2, the temperature rises to 39.6 °C.
- Results
Table 1 demonstrates the control temperatures of Hexamine combusting without an oxidizer.
| Table 1: Control Burn Temperatures | |||
|
| Control 1 | Control 2 | Control 3 |
|
| Sample 1 | Sample 2 | Sample 3 |
| Ambient Temp | 26.0 °C | 25.9 °C | 25.6 °C |
| Max Temp | 172.6 °C | 170.3 °C | 147.2 °C |
| Min Temp | 141.6 °C | 147.8 °C | 129.8 °C |
| Table 2: Enzyme Decomposition Oxidizer Temperatures | |||
|
| Decomp 1 | Decomp 2 | Decomp 3 |
|
| Sample 4 | Sample 5 | Sample 6 |
| Ambient Temp | 25.6 °C | 26.0 °C | 25.4 °C |
| Max Temp | 140.7 °C | 184.2 °C | 175.2 °C |
| Min Temp | 101.9 °C | 103.2 °C | 133.0 °C |
| Table 3: Atomized Oxidizer Temperatures | |||
|
| Atomize 1 | Atomize 2 | Atomize 3 |
|
| Sample 7 | Sample 8 | Sample 9 |
| Ambient Temp | 26.0 °C | 26.0 °C | 25.4 °C |
| Max Temp | 181.0 °C | 156.7 °C | 158.7 °C |
| Min Temp | 91.5 °C | 110.8 °C | 102.9 °C |
After measuring all samples, the highest temperature reaction is the enzyme decomposition. The decomposed hydrogen peroxide from enzyme also produced the highest median temperature.
When considering the mean maximum and minimum of all temperatures in the oxidization on the reaction, the combustion temperature of enzyme decomposed hydrogen peroxide is 11.9 °C less than the control, and the combustion temperature of atomized hydrogen peroxide is 18.0 °C less than the control.
| Table 4: Average Variance in Stability | |
| Method of Introduction | Δ Temperature (Tmax – Tmin) |
| Atomized | 63.7 °C |
| Enzyme | 54.0 °C |
| Control | 23.6 °C |
Additionally, the amount of change between maximum and minimum temperature is 43% less than the control with atomized H2O2; enzyme decomposed H2O2 has a deviation of 37% from the control.
III – Conclusion
In a combustion of Hexamethylenetetramine, hydrogen peroxide as an oxidizer produces the highest maximum temperatures when it is decomposed prior to combustion; for this study pre-decomposition is achieved with the use of enzymes. Including hydrogen peroxide in the combustion process does not increase temperature. In this study hydrogen peroxide must occur prior to combustion to achieve maximum combustion efficiency.
The large variance of average temperatures from methods can be attributed to inconsistent means of introducing the oxidizer to the combustion. In subsequent tests, the oxidizer must be introduced at a uniform and constant rate to decrease the ΔT within the reaction.
Also, it is speculated that the maximum temperatures of a H2O2 will increase when catalyzed with a non-organic compound such as magnesium or silver.
Using industrial strength hydrogen peroxide in future studies could possibly increase combustion efficiencies.
References
[1] Nelson, D. P., and Kiesow, L. A., “Enthalpy of decomposition of hydrogen peroxide by catalase at 25° C (with molar extinction coefficients of H2O2 solutions in the UV),” Analytical Biochemistry, vol. 49, 1972, pp. 474–478.
[2] Berrier, B., “Hydrogen Peroxide Incidents” Available: https://crgis.ndc.nasa.gov/crgis/images/9/95/H2O2.pdf.
[3] Maia, F. F., Gouvea, L. H., Pereira, L. G. F., Vieira, R., and Costa, F. D. S., “Development and Optimization of a Catalytic Thruster for Hydrogen Peroxide Decomposition,” Journal of Aerospace Technology and Management, vol. 6,2014, pp. 61–67.
[4] Ahn, S.-H., Choi, T.-H., Krishnan, S., and Lee, C.-W., “A Laboratory Scale Hydrogen-Peroxide Rocket-Engine Facility,” 39th AIAA/ASME/SAE/ASEE Joint Propulsion Conference and Exhibit, 2003.
[5] Newlands, R. M., Science and Design of the Hybrid Rocket Engine, LULU, 2017.
[6] Dolling, G., and Powell, B. M., “Intermolecular Dynamics of Hexamethylenetetramine,” Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences, vol. 319, 1970, pp. 209–235.
